Services
From conception to patent evaluation
The correct design of pharmaceutical formulations is of enormous importance.
Our services
Concepts
Due to the problems of low solubility, bioavailability and permeability of active pharmaceutical ingredients or instability and incompatibility of active pharmaceuticalingredients (BCS class II-IV) in dosage forms the design of the optimal formulation is today of high importance. These aspects have also to be considered in the re-formulation of known active ingredients.
In order to achieve the optimal design for Your formulation fast and cost efficient IPhaTec starts the development in a small scale (10-500g, non GMP) first by using standard technologies (e.g. Granulation) and modern, innovative technologies (e.g. Nanoparticles, Hot Melt) in parallel.
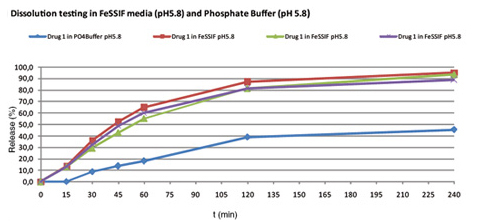
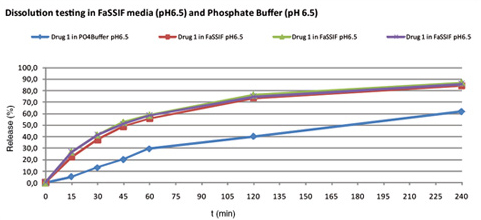
Obtained pre-formulations are analytical tested and suitable pre-formulations will be selected for a first animal trial. However, before these animal trials are conducted, testing of the pre-formulations will be conducted in special biorelevant dissolution media (FaSSGF, FaSSIF, FeSSIF) or precipitation models in-vitro to achieve a better in-vivo prediction and to avoid screening of formulations in animals which are normally expensive.
Technologies
IPhaTec uses not only standard technologies but also modern, innovative technologies for formulation development in addition to the use of standard technologies:
- Hot Melt Technology
- Systems with Nanoparticles
- Silicate Systems with Mesopores
- Amorphous Systems
- Self-emulsifying systems
- Systems with complexes
Consultancies
Reports
Patent evaluations
An important element in the development of new formulation is the possible patent protection.
IPhaTec offers the conduction of a literature research, the drafting of a new patent application by considering the prior-art descriptions and if requested to discuss the patent application with a patent lawyer to come to a final patent application.


